Microcore is an R&D-driven biotech company specializing in the discovery, development, and manufacturing of clinically validated specialty nutraceuticals. With a strong scientific foundation and a commitment to innovation, Microcore focuses on creating evidence-based solutions that address chronic and degenerative health conditions. Evolving from a research-focused organization to a fully integrated, innovation-led global enterprise, Microcore offers end-to-end expertise from concept and formulation to manufacturing and clinical validation. This integrated approach ensures that every product meets the highest standards of efficacy, safety, and regulatory compliance.Microcore develops advanced nutraceuticals targeting a wide range of chronic conditions, including Arthritis & Osteoarthritis, Osteoporosis & Osteopenia, Inflammatory Disorders, Rheumatoid Arthritis, Wound Healing & Tissue Regeneration, Hair Health & Skin Care. Backed by GMP-certified manufacturing, robust clinical research, and a strong intellectual property portfolio, Microcore is committed to improving patient outcomes and enhancing quality of life through science-backed nutraceutical innovation.
It is a great honor to be ranked and chosen amongst an overwhelming number of 37134 nominations filed by SMEs from all over the country and we are delighted to share our happiness with you that MICROCORE is a winner of INDIA SME TOP 100 award 2022

Unit-1 R&D infrastructure, Process development and Production facilities are located at Erode the Textile and Turmeric City of South India. & Unit-2 is under expansion for large scale production of UDCORE-II ( Undenatured Chicken Sternum Collagen Type II) at Tiruchirappalli to Karur. Microcore in-house Research and Development initiatives have been formally recognized by the Department of Scientific and Industrial Research (DSIR), Government of India. With a cumulative experience of over 25 years in research, operations, and manufacturing, the organization has consistently demonstrated a strong commitment to innovation and scientific excellence. Notably, Microcore is the only company to hold PA-II approval for its NESM based product range. This distinction underscores the rigorous quality standards and reflects the comprehensive testing and regulatory compliance its products have successfully met.
![]() No.01/std/PA/FSSAI/2021
No.01/std/PA/FSSAI/2021
![]() No.02/std/PA/FSSAI/2021
No.02/std/PA/FSSAI/2021
![]() No.02/std/PA/FSSAI/2021 (Part 1)
No.02/std/PA/FSSAI/2021 (Part 1)
![]() No.60/std/PA/FSSAI/2022
No.60/std/PA/FSSAI/2022
1.Health Claim :- Microcore NESM promoting the healthy maintenance of joints and connective tissues.
2.Health Claim :- Microcore NESC preventing the risk of Osteoporosis.
![]() CTRI/2021/08/035335 IPRO 344956, IPRO 566050
CTRI/2021/08/035335 IPRO 344956, IPRO 566050
![]() CTRI/2023/02/049901 IPRO 344956
CTRI/2023/02/049901 IPRO 344956
The approval and sanctioning of Microcore products through the Form II application marks a significant regulatory milestone. This achievement supported by trademark registrations, granted patents for both the process and product, and exclusive Clinical Trials Registry India (CTRI) registered clinical studies demonstrates a robust and meticulously documented development pathway for the formulation.Furthermore, the production of these nutraceuticals in facilities certified under Good Manufacturing Practices (GMP), ISO-22000, and Halal standards underscores our commitment to safety, efficacy, and consistent product quality critical pillars in the nutraceutical industry. Microcore is presently focused on marketing its branded bio-actives as ingredients, blends, and finished formulations. The company has also conducted comparative clinical studies evaluating Muttaijow Complex® based nutraceutical combinations versus Diacerin based drug combinations in patients with knee osteoarthritis. These research initiatives highlight strong emphasis on scientific validation, thereby enhancing product credibility and building trust among consumers and healthcare professionals.
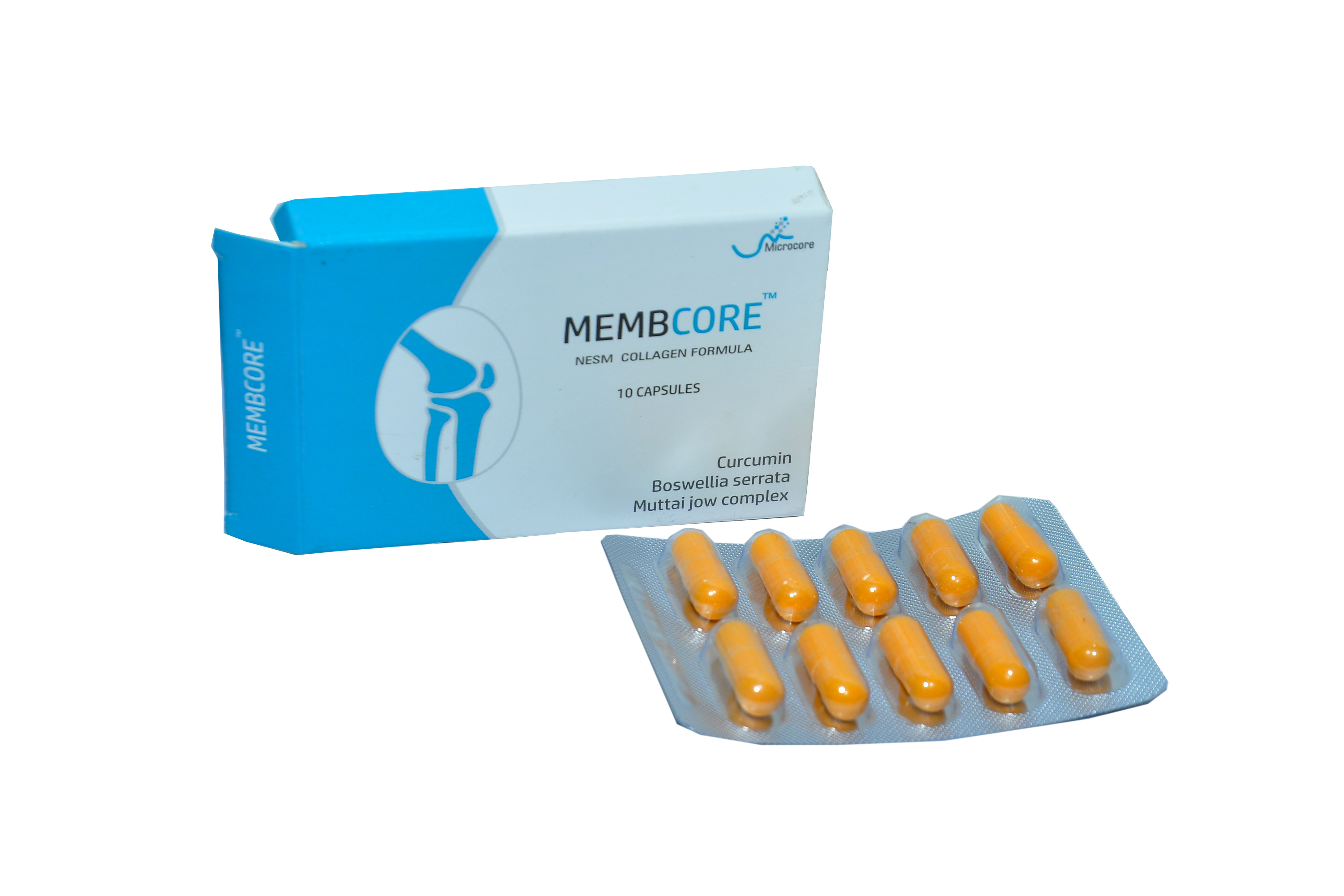
Clinically Studied Branded Bioactives of Microcore
Microcore has branded Chicken Sternum Collagen Type II & NESM that includes UDCORE -ii ® ,NESC®, Muttaijow Complex® & Muttaiodujow® and clinically validated blends for several indications.